- Home
- Metallurgical Coke
Metallurgical Coke
Metallurgical coke or 'Met coke' or 'BF (blast furnace) coke' or simply 'coke in short' is a hard carbon material produced in the process of the 'destructive distillation' of various blends of bituminous coal. It is produced by carbonization of coal at high temperatures (1,100 deg C) in an oxygen deficient atmosphere in a coke oven. Coke is essential for the blast furnace (BF) iron making process in order to support the burden and provide gas permeability, thus a minimum coke burden limit exists. Coke constitutes a major portion of the production costs of hot metal. Other than economical aspects, coke consumption is also strongly related to the CO2 (carbon di-oxide) emissions and hence to the environmental problems.
Production of high-quality coke needed for a modern BF causes the generation of a large amount of small size (under sieve) products namely nut coke and coke breeze (Fig 1). While coke breeze is used as a fuel in the sinter plant, charging of the nut coke into the BF mixed with ore burden has now become a normal practice because of the possibility for coke saving and increase in the furnace productivity.
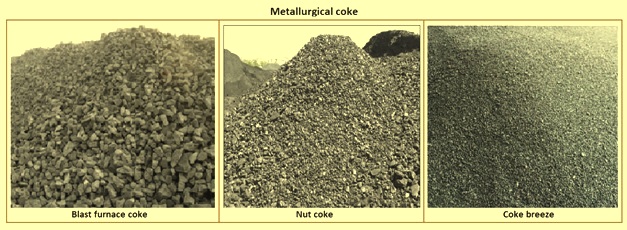
Fig 1 Metallurgical coke
Historical aspects
Coke has ancient origins and carbonization of coal is mentioned in text as early as 371 BCE (Before Common Era). However, coke use as a sole source of fuel in a BF began from somewhere between the early to mid 1,800 century. This coke was made in piles. The knowledge of coke and its properties was lacking in the beginning of the coke era. The higher demands incurred for better pig iron led to higher demands of the coke. During the end of the last century, three consistent themes have appeared pertaining to coke properties and the BF performance. They are related to the viability of the BF, improvement in the BF productivity and efficiency, and BF operations at the lower coke rates.
The BF process of ironmaking, which began in Coalbrookdale in Shropshire in 1709, when Abraham Darby produced pig-iron at the rate of 3 tons per day, using around 4 tons of coke per ton of hot metal has grown to a vast world-wide undertaking, where the BFs are now in operation which are capable of producing at more than 10,000 tons of hot metal/day, using less than 400 kg of coke per ton of hot metal.
Production of BF coke
A good quality coke is generally made from carbonization of good quality coking coals. Coking coals are defined as those coals that on carbonization pass through softening, swelling, and re-solidification to coke. One important consideration in selecting a coal blend is that it should not exert a high coke oven wall pressure and should contract sufficiently to allow the coke to be pushed from the oven. The properties of coke and coke oven pushing performance are influenced by following coal quality and battery operating variables: rank of coal, petrographic, chemical and rheologic characteristics of coal, particle size, moisture content, bulk density, weathering of coal, coking temperature and coking rate, soaking time, quenching practice, and coke handling. Coke quality variability is low if all these factors are controlled.
BF coke is produced by heating coking coal to high temperature in the reducing atmosphere of the by-product coke oven. The coal-to-coke transformation is shown in Fig 2. The coal mix is heated from the oven walls on each side of the coke oven and two plastic layers are developed on each side and converge eventually at the centre of the charge. First, coal near the wall loses water and becomes plastic at 350 deg C to 400 deg C while leaving organic tars. The plastic layers solidify into a semi-coke residue at 450 deg C to 500 deg C while giving off methane and other volatile gases. It takes around 12 hours to 15 hours for the centre of the coke oven to solidify. The semi-coke shrinks and fissures at elevated temperatures of 500 deg C to 1000 deg C losing methane and hydrogen. The coke is pushed out of the coke oven at around 1000 deg C to 1100 deg C, when the volatile matter is less than 1 %. The coke-making process takes around 18 hours to 22 hours, but can vary depending on heating rate and width of the oven.
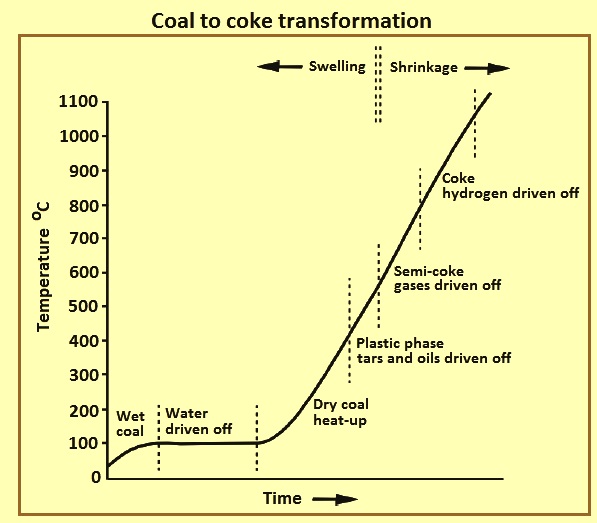
Fig 2 Coal to coke transformation
In case of non-recovery or heat-recovery coke plants the coal is carbonized in large oven chambers. The carbonization process takes place from the top by radiant heat transfer and from the bottom by conduction of heat through the sole floor. Primary air for combustion is introduced into the oven chamber through several ports located above the charge level in both pusher and coke side doors of the oven.
The water content in coke is practically zero at the end of the coking process, but it is often water quenched so that it can be transported to the blast furnaces. The porous structure of coke absorbs some water, usually 3 % to 6 % of its mass. In some of the coke plants dry quenching of coke is practiced. Coke is normally available in 3 varieties. These are coke breeze (size -10 mm), nut coke (size +10 mm to –25 mm) and blast furnace (BF) coke (+25 mm to -80 mm).
Characteristics of the coke
Coke is a high-carbon product. High-quality BF coke is to have low and consistent moisture content, low content of impurities, high strength, relatively low reactivity and a uniform size range. These characteristics of the coke are mainly governed by a proper mix of suitable coals and the optimization of the coking conditions in the coke ovens such as coal size, bulk density and coke oven heating rate.
The quality of coke is determined in a multi-parametric manner. The first is by determining the percentage of analytical component (moisture, ash, volatile matters and total carbon), and recently alkaline compounds. The second is the evaluation of physical properties such as density, porosity, and grain composition. The third is the determination of its mechanical properties, such as strength and abrasiveness. Sometimes its heating value (calorific value) is additionally determined. In addition, two additional parameters (CRI and CSR) of the coke are also assessed.
Coke serves multiple roles in the BF. It acts as a reducing agent and source of reducing CO (carbon mono-oxide) gas, a source of heat, a filter of dust and soot, a carburizer of hot metal, and as a structural support material. Its role as a structural support material is especially important, particularly in the lower parts of the BF, since it cannot be replaced by any other raw materials. A lack of permeability in the BF restricts the blowing rates and leads to poor gas distribution in the shaft area. The flow of fluids in the lower BF is also strongly influenced by mean particle size and voidage of the material bed.
The most important characteristics of coke regarding permeability are an optimal size, good pre- reaction and post-reaction strengths and a narrow size range. Poor coke characteristic leads to excessive size degradation and the formation of fines, both of which can impair the permeability of the BF. The permeability of the coke bed in the lower part determines the technological limits of the BF, including maximum driving rates, best fuel efficiency and long campaign life.
Poor coke characteristics can result in the adverse effects in the BF namely (i) increased flue dust, (ii) changes to the shapes of the raceway and cohesive zones, (iii) increased heat losses, (iv) the channelling of flow of liquids and solids, (v) increased pressure loss, and (vi) decreases in the drainage ability of the deadman. An overview different stresses in the BF and the effects of poor coke characteristics on the BF performance are depicted in Fig 3.
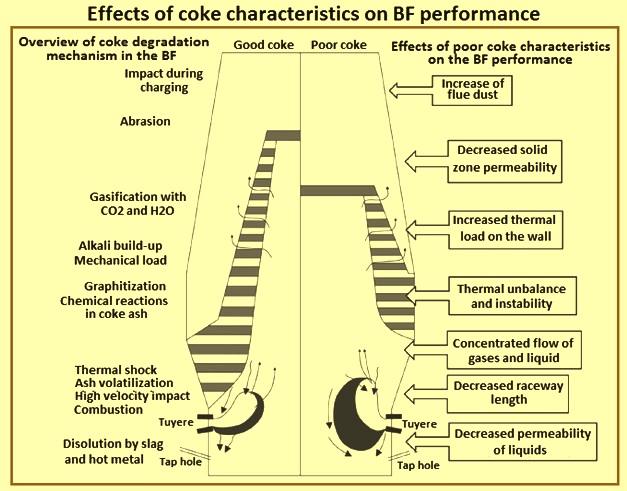
Fig 3 Effects of coke characteristics on the BF performance
The coke is one of the very important input materials for the BF process of the ironmaking. It performs three important functions (Fig 4) in a BF namely (i) a thermal function, as fuel providing the energy required for endothermic chemical reactions and for melting of iron and slag, (ii) a chemical function, as reductant by providing reducing gases for iron oxide reduction, and (iii) a mechanical function, as a permeable grid providing for the passage of liquids and gases in the furnace, particularly in the lower part of the furnace. When coke passes through the BF, the coke degrades and generates fines which affect bed permeability and affects the process efficiency. The rate at which coke degrades is mainly controlled by the solution loss reaction, thermal stress, mechanical stress and alkali accumulation.
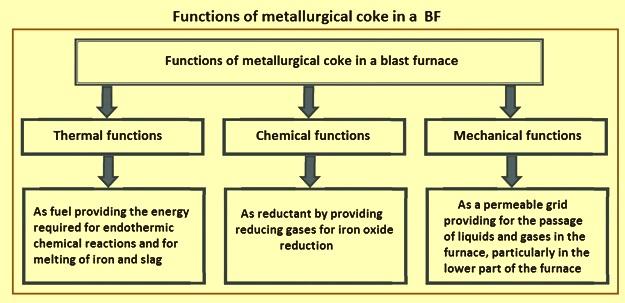
Fig 4 Functions of BF coke in a BF
BF operation with low coke rate and high injection rate causes a change in the coke characteristics. Some of its functions become less important (heat source, reducing agent) and its other functions (maintenance of gas permeability, resistance against alkali attack and metal carburization) on the contrary become decisive
From a chemical point of view, coke has a rather simple composition, consisting of predominately carbon with some mineral matters and minor amounts of hydrogen, sulphur and oxygen. High ash content of the coke can be a problem, mainly for economy, since the BF fuel rate increases and the productivity decreases. It affects the heat balance of the BF in two ways namely (i) by lowering the carbon content in coke, which lowers calorific value, and (ii) by requiring additional slag former to melt the coke ash, which demands extra energy and increases the slag volume. Alkalis in coke ash are normally present as silicates and are the largest source of alkalis in BF burden, as much as 75 % of alkali input comes from coke.
Coke is very complex structurally, with a variety of pore and wall sizes and shapes as well as fissures. The coke walls consist of different textures which have diverse microscopic properties and vary in optical anisotropy depending on the rank and type of coal in the coal mix used. Simplified, coke carbon consists of moderate crystallized anisotropic parts and amorphous isotropic parts. The anisotropic parts can be divided into 'mosaic' and 'flow type' (Tab 1). Coke with higher degrees of anisotropic texture shows generally lower reactivity (CRI) and higher coke strength after reaction (CSR). Isotropic parts have generally higher reactivity towards CO2, which increases the CRI value.
| Tab 1 Relationship between carbon texture and coke properties | ||||
| Sl. No. | Coal texture | Isotropic | Mosaic | Flow-type |
| 1 | Mechanical strength | Weak | Medium | Strong |
| 2 | Resistance to CO2 attack | Weak | Strong | Medium |
| 3 | Resistance to alkali attack | Strong | Medium | Weak |
Coke quality is often characterized by measuring cold and hot strength, ash composition and chemistry, which are largely dictated by coal properties. A range of laboratory tests and procedures have been developed to characterize physical and chemical properties of coke and their potential impacts in the BF operations. The most often used and well-known tests are the Coke Reactivity Index (CRI) and the Coke Strength after Reaction (CSR) developed by Nippon Steel Corporation (NSC) in Japan in the early seventies, in order to assess the effect of CO2 reactions on coke. There is no universally accepted standard procedure, however NSC/CRI test is widely recognized around the world and was adopted by other standard. Normally high CSR coke is believed to prevent the coke from breaking down, improve the permeability of gas and liquid and increase the productivity as well as decrease the specific coke consumption of the BF.
Uses of metallurgical coke
Besides being used in BF, sinter production, steelmaking furnaces and ferro – alloy production, metallurgical coke has many more applications. It is used where a tough and resilient, high quality wearing carbon is needed. Metallurgical coke's applications include for example: friction materials, conductive flooring, foundry coatings, corrosion materials, foundry carbon raiser, reducing agents, drilling applications, ceramic packing media, heat-treatment, oxygen exclusion and electrolytic processes. Metallurgical coke can be also used as a filler coke for the poly-granular carbon products.
- CONTACT US +86-13526138080 mingshancoke@163.com China Sales Office: R1108, 11TH FLOOR, No.8 BUILDING, HUAQIANG CENTER, ANYANG, CHINA. LONDON OFFICE: RM101, MAPLE HOUSE, 118 HIGH STREET, PURLEY LONDON, ENGLAND, UNITED KINGDOM, CR8 2AD.
- Production Capacity 1. Metallurgical coke (powder) production capacity of 100,000 tons/month 2. Silicon carbide ball processing 6000 tons/month 3. Semi coke production capacity of 5,000 tons/month 4. Ferrosilicon processing capacity of 50,000 tons/month Related Products 1. High Carbon Ferro Silicon 2. Silicon Metal 3. Silicon Metal Powder 4. Silicon Briquette
- GET IN TOUCH
 العربية
العربية Français
Français Español
Español English
English



